

Neurocritical Care Physiology I:
Normal intracranial pressure measures 5-12mmHg.
Intracranial hypertension is defined by ICP >20mmHg.
-
At ~30mmHg, focal areas of cerebral ischaemia can form
-
At >40mmHg global ischaemia can develop and herniation can occur.
Raised ICP following head trauma has been associated with poor neurological outcomes in multiple retrospective cohorts.
This is one of the reasons why measuring ICP in severe brain injury is recommended by the Brain Trauma Foundation consensus guidelines.

This curve named the Munro-Kellie Doctrine, after the two Scotsmen who published it in 1783-1824, demonstrates the relationship between intracranial volume and ICP.
An increase in intracranial volume produces a small change in intracranial pressure when initial intracranial volume is low and compensatory mechanisms are not exhausted. However, when compensatory mechanisms are exhausted, similar increases in intracranial volume result in large increases in ICP.
i.e. There is a non-linear change in pressure per unit change in volume.

Why
Once the fontanelles and sutures have closed, the brain is enclosed in a non-expandable case of bone with an internal volume ~1500ml.
Firm dural structures support the brain and further subdivide the cranial cavity into fossae.
The tentorium is a tent-like fibrous dural structure forming the semi-open roof of the posterior fossa.
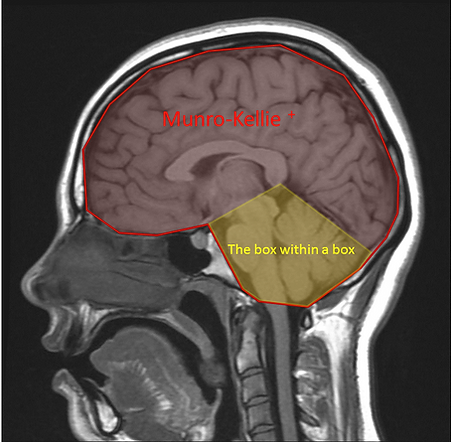
The posterior fossa containing the brainstem and cerebellum, behaves as a box within a box and is subject to very limited compensation. Urgent neurosurgical intervention is required for most acute pathology.
The brain parenchyma is nearly incompressible acutely without ischaemia or herniation from one dural compartment into another.
Brain parenchymal volume ~1200ml, is regulated in health by the crystalloid osmotic pressure gradient (predominantly determined by Na+ concentrations) across an intact semi-permeable blood-brain barrier.
Intracranial pressure is regulated acutely by:
-
Venous capacitance (static volume ~70ml, dynamic volume ~700ml/min)
-
Arterial smooth muscle tone (static volume 30-60ml, dynamic volume 700ml/min)
-
Cerebrospinal Fluid (static volume ~75ml, dynamic volume 0.35ml/min)
Acute compensation is provided by dynamic venous capacitance and to a lesser extent arterial autoregulation (see Physiology II: Cerebral Blood Flow).
ICP = P + P + CSF x R
Vascular component derived from pulsation of arterial bed
Sagittal sinus
Formation rate
CSF
Acute
Chronic
An adaption of Davson's formula; where P= pressure (mmHg); CSF formation rate (ml/min);R is the resistance to CSF outflow(mmHg/ml/min)
CSF
The importance of adequate venous drainage

The venous drainage of the brain demonstrates marked heterogeneity between individuals.
Most exhibit a dominant right sided drainage into the internal jugular system. Excessive rotation of the head or the overexuberant application of a hard cervical collar can result in impaired venous drainage and subsequently reduce venous capacitance, increasing ICP by up to 15mmHg.
Many have discrete asymptomatic stenoses resulting in reduced venous capacitance.
Some of these patients will develop benign intracranial hypertension as a result.
Even in health, those with reduced venous capacitance may be more susceptible to high altitude cerebral oedema.
The bridging veins, draining the parenchyma into the veins labelled in the diagram above, are the site of ICP and cerebral venous pressure coupling.
Continuous outflow of venous blood is required to make room for continuous arterial blood inflow.
Any compromise of venous outflow, either by intracranial or extracranial means, will result in a rapid increase in ICP.
This important concept was highlighted by the Neurosurgeon and HEMS doctor Mr Mark Wilson:
INTRACRANIAL vs. EXTRACRANIAL causes of compromised venous outflow:

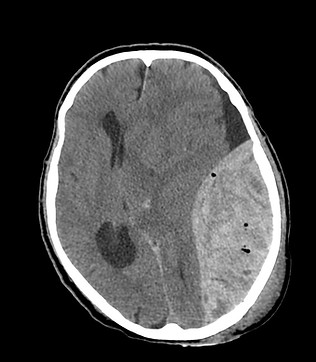
All biological tissues can tolerate strain better if deformed slowly rather than quickly.
i.e. any compensation for volume change is time dependent.
The compensation for a 150ml slow-growing tumour will be more effective than for a rapidly-expanding 150ml haematoma, as seen in the CT images below:

The role of Cerebrospinal Fluid (CSF)

CSF is produced by the choroid plexus in both the lateral and 4th ventricles at a rate of 0.35ml.min or ~500ml/day.
Small channels between the ventricles or foramen allow the pulsatile flow of the CSF circulation. These foramen can become compressed by extrinsic pressure from a space occupying lesion or blocked by debris or blood. The accumulation of trapped CSF within an isolated ventricular system is known as hydrocephalus.
CSF is reabsorbed within the cranium through the arachnoid granulations that penetrate the walls of the sagittal sinus. CSF drainage will cease if the subarachnoid intracranial pressure is less than sagittal sinus pressure. This can be seen clinically when a hygroma forms following a crainectomy.
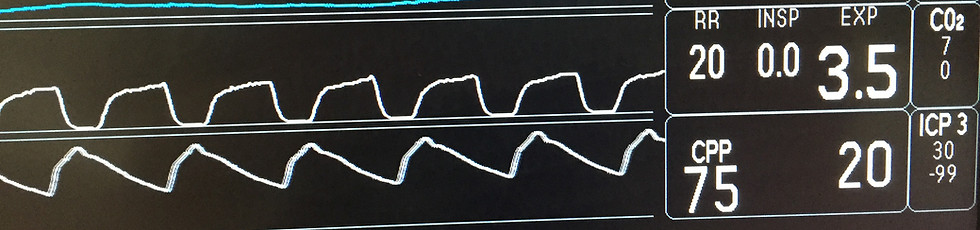
ICP is much more than just a number!
The ICP waveform can be measured via an external ventricular drain or more commonly in the UK via a parenchymal strain gauge.
The intracranial pressure waveform is composed of numerous dynamic variable components.
The pulsatile triphasic pattern reflects transmitted pressure changes in the diameter of the arachnoid vili arterioles combined with a dynamic component indicating the intracranial compliance. For further information see APPENDIX 3 of the Traumatic Brain Injury Protocol.
Lundberg waves
Nils Lundberg described a series of low frequency vasogenic waves with frequencies typically below that at which digital monitors can discriminate.
As a result, Lundberg waves are an often overlooked and underreported explanation for a cyclical pattern of intracranial pressure.
A
Plateau waves. Magnitude 50-100mmHg. Lasting 5-20minutes. Always pathological.
They are a sign of impending tonsillar herniation with brainstem compression and are associated with a classical Cushing response.
Cerebral perfusion pressure is inadequate to meet metabolic demand resulting in a dramatic loss of autoregulation. This results in profound arterial vasodilatation increased cerebral blood volume, triggering a further rise in ICP and a further reduction in CPP until death ensues.
B
Oscillations 10-50mmHg. 0.5 to 2/min. They reflect vasomotor instability when CPP is unstable or at the lower limits of autoregulation.
C
Up to 20mmHg. 4-8/min. Seen in health. Interaction between cardiac and respiratory cycles,
How does all this fit together at the bedside?
Treatment decisions should never be based on the ICP number in isolation.
The ICP trend over the last 24-48 hours, ICP waveform interpretation and the dynamic interaction of ICP with PaCO2, PaO2, Bp and sometimes the response to transient compression of the neck veins are all useful to inform decision making.
Sound clinical neurological examination and interpretation of the appearances seen on CT/MRI imaging are vital components of quality neurocritical care.
This video of Randy Chestnut (lead author of the BEST TRIP trial) speaking at the Neurotrauma 2015 meeting in London will help to consolidate Physiology I: Munro-Kellie+ and gives some clinical context.